so2-3 lewis structure|SO2 Lewis Structure : Baguio Lewis Structure of SO2 (sulfur dioxide) chemistNATE. 276K subscribers. Subscribed. 7K. 949K views 9 years ago. How to draw the Lewis Structure of SO2 - with . Spielen Sie den besten Online-io-Spiele kostenlos auf CrazyGames, kein Download oder Installation erforderlich. 🎮 Spiele jetzt Bloxd.io und viele mehr!
so2-3 lewis structure,A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons .
Lewis Structure of SO2 (sulfur dioxide) chemistNATE. 276K subscribers. Subscribed. 7K. 949K views 9 years ago. How to draw the Lewis Structure of SO2 - with .
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.The Lewis structure of SO3 2- represents the arrangement of atoms and valence electrons in the sulfite ion. It shows one sulfur (S) atom bonded to three oxygen (O) atoms, with an overall -2 charge on the ion. The Lewis structure .
This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and .
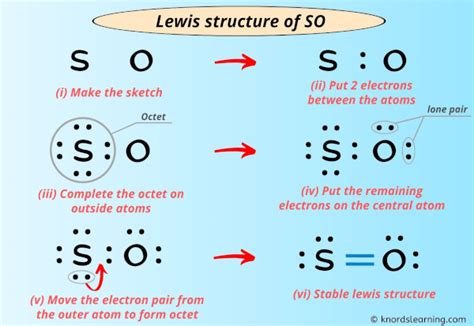
so2-3 lewis structure SO2 Lewis Structure To draw the SO2 Lewis structure, follow these simple steps: 1. Determine the total valence electrons. Start by counting the valence electrons of each atom in the molecule. In SO2, sulfur is in Group 6, so it has 6 valence electrons, while .
SO2 (Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry, and Bond Angles. The chemical formula SO2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a .

The Lewis structure for SO 3 2-is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 3 at first. Remember, .
Certain cookies and other technologies are essential in order to enable our Service to provide the features you have requested, such as making it possible for you to access our product and .What is the structure of SO 2?I have seen two different ways the Lewis Structure is written: The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. So I would . The SO 2 Lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms. In the SO 2 Lewis structure, there is a double bond between the sulfur atom and each .so2-3 lewis structureAround sulfur atom, there are four bonds and a single lone pair in the lewis structure of SO 3 2-ion. Therefore, five electron groups are around the central atom of SO 3 2-ion. Is there are charges on sulfur atom in sulfite ion lewis .
SO2 Lewis Structure A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3 2- Lewis structure the total number of valence electrons .
What is the Lewis structure of [//substance:SO2//]? Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, mathematics, linguistics, sports . 6 Steps to Draw the Lewis Structure of SO2 Step #1: Calculate the total number of valence electrons. Here, the given molecule is SO2 (sulfur dioxide). In order to draw the lewis structure of SO2, first of all you have to find the total number of .
The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species. These bonds can be single, double, or triple.
Question: SO2−3 Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons and nonbonding electrons. Show the formal charges of all atoms in the correct structure. SO2−3. A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth. Steps for drawing the SO2 Lewis structure. Step 1 Calculate the number of valence electrons for S and O. Sulphur and oxygen are both elements of group 16 on the periodic table. Therefore, there are 6 valence electrons in both sulphur and oxygen atoms, so the total valence electrons in the SO2 molecule = valence electrons given by 1 sulphur atom . Steps of drawing SO2 lewis structure Step 1: Find the total valence electrons in SO2 molecule. In order to find the total valence electrons in SO2 (sulfur dioxide) molecule, first of all you should know the valence .Draw the Lewis Structure for the chlorate ion (ClO 3-). Solution. First, lets find the how many valence electrons chlorate has: ClO 3 - : 7 e-(from Cl) + 3(6) e-(from 3 O atoms) + 1 (from the total charge of -1) = 26 . There are 26 valence electrons. Next . The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom . {O-SO2}\), and the resonance structures) \(\ce{NO3-}\) (see Example 2 below) \(\ce{CO3^2-}\) (ditto) Notice that some of the resonance structures may not satisfy the octet rule. The \(\ce{NO2}\) molecule has .
A plot of the potential energy of the system as a function of the internuclear distance (Figure 5.3.2 ) shows that the system becomes more stable (the energy of the system decreases) as two hydrogen atoms move toward each other from r = ∞, until the energy reaches a minimum at r = r 0 (the observed internuclear distance in H 2 is 74 pm).Thus at intermediate distances, .

Lewis Structure of SO2. A sulfur atom (S) and two oxygen atoms (O) make up the SO2 Lewis structure. The sulfur atom (S) is the center atom, and the two oxygen atoms (O) surround it at a bond angle of 119 degrees. The sulfur atom (S) and each oxygen atom (O) form two double bonds. The two oxygen atoms (O) each have two lone pairs, while the .
Lewis Structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Hence, Lewis Structure is also commonly called Electron Dot Structure. Let us proceed to draw the most appropriate LS diagram of CO32- ion. Step 1: Count the Total Number of Valence Electrons.The Lewis structure for SO 3 2-is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 3 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. You'll want to calculate the formal charges on each atom to make sure you have the best Lewis . SO2 = 18 Valence Electrons SO2 Lewis Structure Setup Step-3: Now we have to determine the central atom in SO2.The central atom is that kind of atom that is single or that has lower electronegativity.In case of SO2, S is the central atom and oxygen ,O, is the outer atom as sulfur is less electronegative than than O.
so2-3 lewis structure|SO2 Lewis Structure
PH0 · SO3 2
PH1 · SO2(Sulfur Dioxide) Lewis Structure, Hybridization,
PH2 · SO2 Lewis Structure, Hybridization, Molecular Geometry, and
PH3 · SO2 Lewis Structure
PH4 · SO2 (Sulfur Dioxide) Lewis Structure
PH5 · SO 3 2
PH6 · Lewis Structure of SO2 (sulfur dioxide)
PH7 · Lewis Structure Finder
PH8 · Khan Academy